After obtaining the approval of the "Class II Registration Certificate for Medical Devices", with the approval of the Shandong Provincial Drug Administration, Lixin Nuokang (Shandong) Biomedical Technology Co., Ltd., a wholly-owned subsidiary of Linaxin Biotechnology, has been granted a Class II medical device production license. The production scope is Class II: II-6840 in vitro diagnostic reagents, and the license number is Luyao Jianxie Production License No. 20210149.
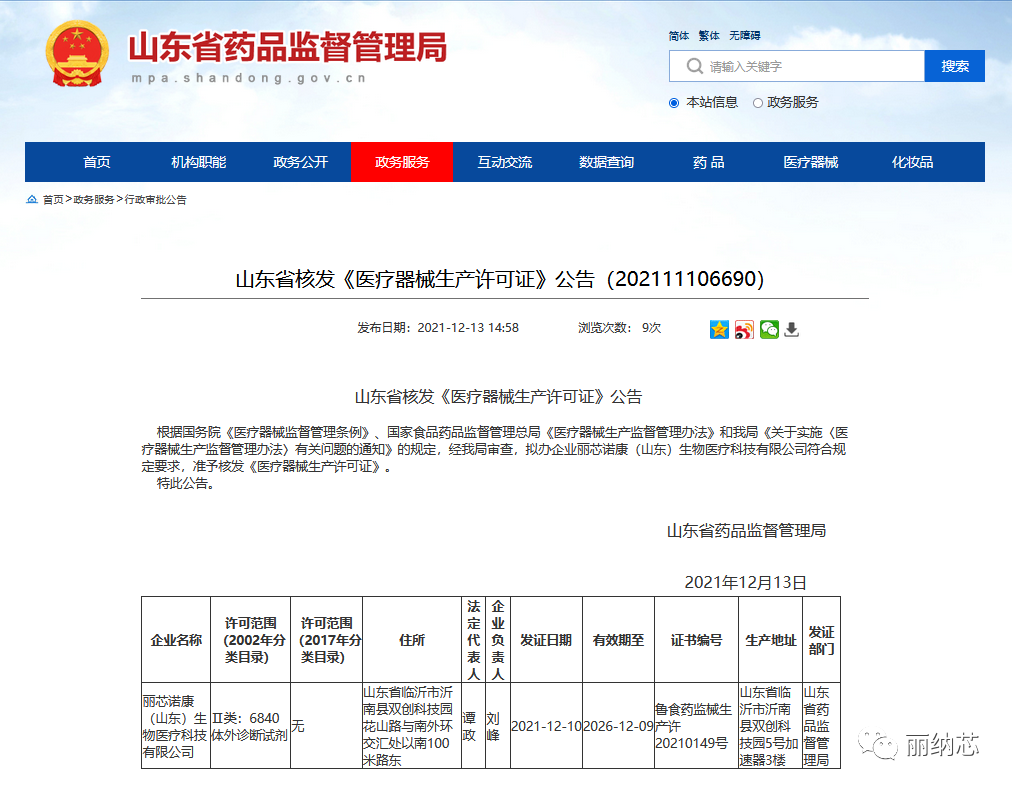
Previously, Lixin Nuokang (Shandong) Biomedical Technology Co., Ltd. had obtained the production record certificate for Class I medical devices, with a production scope of Class I: 22 clinical testing devices, and record numbers: Lulin Medical Equipment Preparation 20210060, Lulin Medical Equipment Preparation 20210059, Lulin Medical Equipment Preparation 20210113. This time, we have obtained the production license for Class II medical devices, which means that Linaxin Biotechnology's production equipment, professional technical personnel, quality management system, after-sales service capabilities, and other aspects meet the requirements of relevant laws and regulations, and have all the conditions for the production of Class II medical devices. This marks another big step forward for the company in the field of medical devices. At the same time, the fecal occult blood detection kit (ChanglexinTM), nucleic acid extraction and purification kit, fecal gene detection pretreatment device, and fecal colon cancer detection cell preservation solution independently developed by Linaxin Biotechnology can all be officially put on the market for sale!
When the wind is strong, one should set sail and break through the waves; The road ahead is long and arduous, and we still need to ride a horse and whip. Linaxin Biotechnology will continue to focus on customers, continuously improve the company's product and service capabilities, strive to practice Linaxin Biotechnology's corporate culture, and continue to build Linaxin Biotechnology's core competitiveness.
100000 level dust-free purification in vitro diagnostic reagent production base
The dust-free workshop is designed strictly in accordance with the ISO13458 system standards, introducing a standard matched air purification treatment system to create a fully enclosed standard production environment. The raw materials are sterilized and disinfected by ultraviolet radiation, and each production process is monitored in real time to ensure the high-quality standards of the products.
Equipped with a 10000 level microbiology laboratory and an international standard physical and chemical laboratory, the testing projects cover bacterial filtration efficiency, particle filtration efficiency, water quality indicators, microbiological indicators, raw material quality control, pressure steam sterilization, finished product quality control, etc., providing technical support for the production of high-quality in vitro diagnostic reagents.







![]()


早筛一个样本,挽救一个生命,造福一个家庭,提升人民健康水平!
Domestic Solid-State Nanopore Sequencing Makes a B...2025-11-04

Solid-State Nanopore Technology Usheres in a Criti...2025-11-04

Linaxin collaborates with Linyi Working Committee ...2025-07-24

Linaxin and Zhejiang Qianjifang have reached a str...2025-07-24

The project of Linaxin and Zheng Zheng Hospital to...2025-07-24

 WeChat service account
WeChat service account