On November 10th, a portable fecal occult blood test kit independently developed by Linaxin Biotechnology successfully obtained the Class II medical device product "Medical Device Registration Certificate" issued by the Shandong Provincial Drug Administration. As one of the approved products with independent property rights owned by Linaxin Biotechnology, this product is produced by Linaxin's wholly-owned subsidiary, Linaxin Nuokang. The successful acquisition of the medical device registration certificate for this product fully reflects the strong technological research and development capabilities of Linaxin Biotechnology in the field of in vitro diagnosis of medical devices, and also represents the high recognition of regulatory authorities for Linaxin Biotechnology's quality and safety, production management capabilities, and other aspects.
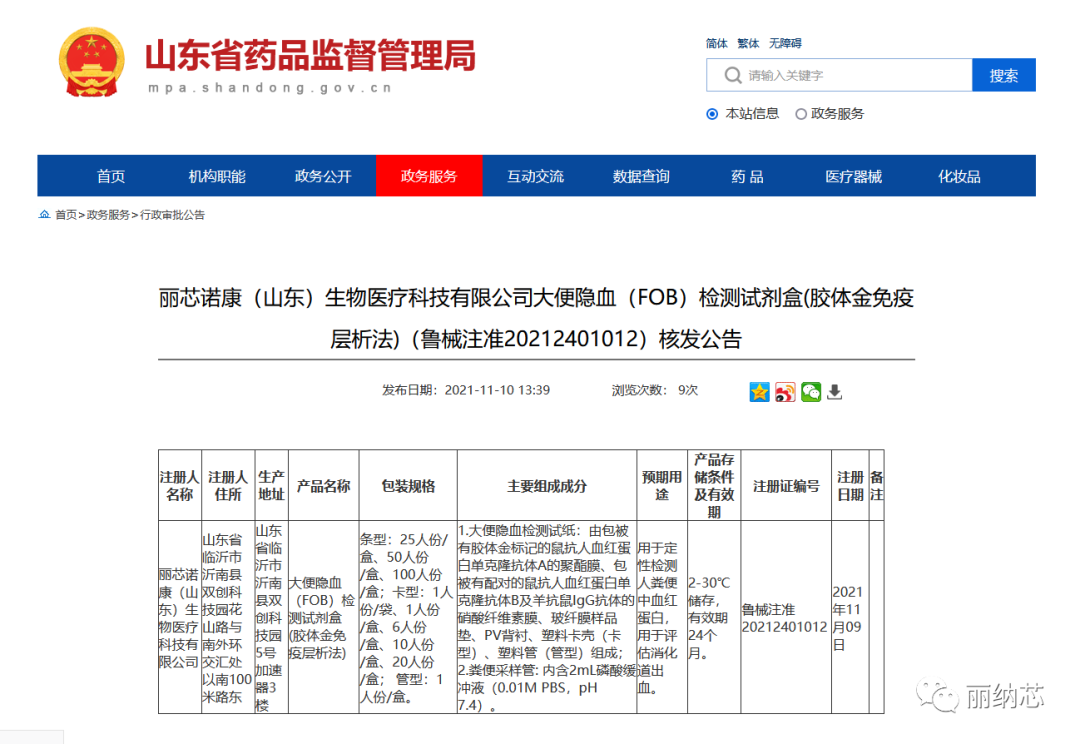
With the development of society and the improvement of residents' health awareness, the field of early screening, early treatment, and early prevention of cancer has developed into a blue ocean. Linaxin Biotechnology has long been committed to solving the clinical pain points of today's early screening technology, providing users with more accurate, portable, efficient, and home-based early cancer screening detection products. This successful acquisition of the "Medical Device Registration Certificate" for Class II medical device products has laid a solid foundation for the design and development of a series of digestive tract related early cancer screening products that the company is currently developing. It has a positive driving significance for the company's exploration in the field of medical home early screening.
Domestic Solid-State Nanopore Sequencing Makes a B...2025-11-04

Solid-State Nanopore Technology Usheres in a Criti...2025-11-04

Linaxin collaborates with Linyi Working Committee ...2025-07-24

Linaxin and Zhejiang Qianjifang have reached a str...2025-07-24

The project of Linaxin and Zheng Zheng Hospital to...2025-07-24

 WeChat service account
WeChat service account